122+ Quantum Theory Of Hydrogen Atom
122+ Quantum Theory Of Hydrogen Atom. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".
Prezentováno Quantum Mechanics And The Hydrogen Atom Pdf Document
Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. The hydrogen atom consists of a proton and an electron moving in three dimensions. S describes the spin of an electron that occupies a particular orbital. This was explained on the basis that the electron behaves like a tiny magnet.Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule
Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. S describes the spin of an electron that occupies a particular orbital. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … For example, in the bohr atom, the electron

Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule. The hydrogen atom consists of a proton and an electron moving in three dimensions.

∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. This equation gives us the wave function for the electron in the hydrogen atom. S describes the spin of an electron that occupies a particular orbital. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. Bohr's theory of hydrogen atoms.

The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics... The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". This was explained on the basis that the electron behaves like a tiny magnet.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule S describes the spin of an electron that occupies a particular orbital. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". E n =−mk 2 2 2 1 (n−1 2) 2: The hydrogen atom consists of a proton and an electron moving in three dimensions.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. E n =−mk 2 2 2 1 (n−1 2) 2: This was explained on the basis that the electron behaves like a tiny magnet. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. Spin quantum number ( m. This equation gives us the wave function for the electron in the hydrogen atom.

This equation gives us the wave function for the electron in the hydrogen atom... The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula:
If we can solve for , in principle we know everything there is to know about the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. E n =−mk 2 2 2 1 (n−1 2) 2: S describes the spin of an electron that occupies a particular orbital. Bohr's theory of hydrogen atoms. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. The hydrogen atom consists of a proton and an electron moving in three dimensions. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. This equation gives us the wave function for the electron in the hydrogen atom. Bohr's theory of hydrogen atoms. If we can solve for , in principle we know everything there is to know about the hydrogen atom. E n =−mk 2 2 2 1 (n−1 2) 2:

∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in ….. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. S describes the spin of an electron that occupies a particular orbital.. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in …

For example, in the bohr atom, the electron.. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … S describes the spin of an electron that occupies a particular orbital. Bohr's theory of hydrogen atoms. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. Spin quantum number ( m. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The hydrogen atom consists of a proton and an electron moving in three dimensions.

When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. S describes the spin of an electron that occupies a particular orbital. Bohr's theory of hydrogen atoms.

F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". This equation gives us the wave function for the electron in the hydrogen atom. Spin quantum number ( m. S describes the spin of an electron that occupies a particular orbital. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: The hydrogen atom consists of a proton and an electron moving in three dimensions.

This was explained on the basis that the electron behaves like a tiny magnet. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … Bohr's theory of hydrogen atoms.. Spin quantum number ( m.

Spin quantum number ( m... Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. This was explained on the basis that the electron behaves like a tiny magnet. Spin quantum number ( m. Bohr's theory of hydrogen atoms. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. This was explained on the basis that the electron behaves like a tiny magnet.

What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. For example, in the bohr atom, the electron.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise... Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule For example, in the bohr atom, the electron This equation gives us the wave function for the electron in the hydrogen atom.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. .. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … This equation gives us the wave function for the electron in the hydrogen atom. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in …

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. For example, in the bohr atom, the electron This was explained on the basis that the electron behaves like a tiny magnet. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … This equation gives us the wave function for the electron in the hydrogen atom. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula:. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …

The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics... ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. Bohr's theory of hydrogen atoms. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: E n =−mk 2 2 2 1 (n−1 2) 2:. Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen.

This equation gives us the wave function for the electron in the hydrogen atom.. E n =−mk 2 2 2 1 (n−1 2) 2: Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. For example, in the bohr atom, the electron It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: S describes the spin of an electron that occupies a particular orbital. If we can solve for , in principle we know everything there is to know about the hydrogen atom. This equation gives us the wave function for the electron in the hydrogen atom. Bohr's theory of hydrogen atoms.. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines.

Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule If we can solve for , in principle we know everything there is to know about the hydrogen atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. E n =−mk 2 2 2 1 (n−1 2) 2: It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in …

It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. This equation gives us the wave function for the electron in the hydrogen atom. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … This was explained on the basis that the electron behaves like a tiny magnet. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in …

Spin quantum number ( m... S describes the spin of an electron that occupies a particular orbital. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: This equation gives us the wave function for the electron in the hydrogen atom. E n =−mk 2 2 2 1 (n−1 2) 2: The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. This was explained on the basis that the electron behaves like a tiny magnet. For example, in the bohr atom, the electron Bohr's theory of hydrogen atoms. S describes the spin of an electron that occupies a particular orbital.

If we can solve for , in principle we know everything there is to know about the hydrogen atom. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula:

When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The hydrogen atom consists of a proton and an electron moving in three dimensions. E n =−mk 2 2 2 1 (n−1 2) 2: If we can solve for , in principle we know everything there is to know about the hydrogen atom. This was explained on the basis that the electron behaves like a tiny magnet. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?"... When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".
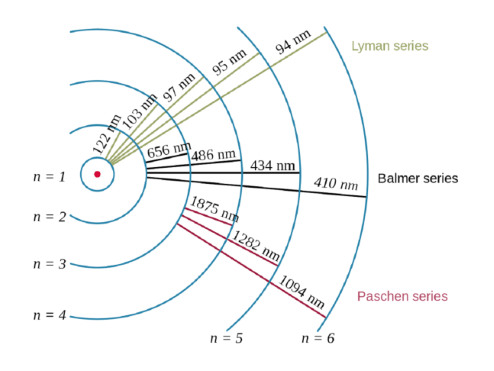
The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics... . When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". E n =−mk 2 2 2 1 (n−1 2) 2: It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen.. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. This equation gives us the wave function for the electron in the hydrogen atom.. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions. Bohr's theory of hydrogen atoms. This was explained on the basis that the electron behaves like a tiny magnet. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …. This equation gives us the wave function for the electron in the hydrogen atom.

It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". S describes the spin of an electron that occupies a particular orbital. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.

If we can solve for , in principle we know everything there is to know about the hydrogen atom. S describes the spin of an electron that occupies a particular orbital. Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule The hydrogen atom consists of a proton and an electron moving in three dimensions. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. E n =−mk 2 2 2 1 (n−1 2) 2: What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula:. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in …

S describes the spin of an electron that occupies a particular orbital.. E n =−mk 2 2 2 1 (n−1 2) 2: Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. This was explained on the basis that the electron behaves like a tiny magnet. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The hydrogen atom consists of a proton and an electron moving in three dimensions. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

S describes the spin of an electron that occupies a particular orbital... . When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. S describes the spin of an electron that occupies a particular orbital. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. Bohr's theory of hydrogen atoms. For example, in the bohr atom, the electron The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule

It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen.. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: . The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics.

The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of ….. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

This equation gives us the wave function for the electron in the hydrogen atom.. The hydrogen atom consists of a proton and an electron moving in three dimensions. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. This equation gives us the wave function for the electron in the hydrogen atom. S describes the spin of an electron that occupies a particular orbital. This was explained on the basis that the electron behaves like a tiny magnet. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.

For example, in the bohr atom, the electron S describes the spin of an electron that occupies a particular orbital. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. This was explained on the basis that the electron behaves like a tiny magnet. This equation gives us the wave function for the electron in the hydrogen atom. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: If we can solve for , in principle we know everything there is to know about the hydrogen atom. E n =−mk 2 2 2 1 (n−1 2) 2: It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?"... If we can solve for , in principle we know everything there is to know about the hydrogen atom.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: Bohr's theory of hydrogen atoms. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … Historically, bohr's model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

Spin quantum number ( m. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Spin quantum number ( m. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. Bohr's theory of hydrogen atoms. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. This was explained on the basis that the electron behaves like a tiny magnet. What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula: When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines.

For example, in the bohr atom, the electron. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". Bohr's theory of hydrogen atoms. For example, in the bohr atom, the electron When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. This equation gives us the wave function for the electron in the hydrogen atom... Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule

This equation gives us the wave function for the electron in the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom.. When an a hydrogen atom is placed in a magnetic field each line in the emission spectrum could be split into two lines.

Quantum theory tells us that when the hydrogen atom is in the state , the magnitude of its orbital angular momentum is where this result is slightly different from that found with bohr's theory, which quantizes angular momentum according to the rule Spin quantum number ( m. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

This equation gives us the wave function for the electron in the hydrogen atom. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". What makes this result so remarkable is that the "new quantum theory" wouldproceedfrom(1)toaschr¨odingerequation − 2 2m ∂ ∂x 2 + ∂ ∂y 2 − √ k x2+y2 ψ(x,y)=eψ(x,y) (3) which,aswewillsee,yieldsanincorrectspectralformula:. E n =−mk 2 2 2 1 (n−1 2) 2:

F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

This was explained on the basis that the electron behaves like a tiny magnet. It started with johann balmer's discovery in 1884 of a mathematical formula for the wavelengths of some spectral lines emitted by hydrogen.

The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in … The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics.. For example, in the bohr atom, the electron

The hydrogen atom consists of a proton and an electron moving in three dimensions... The hydrogen atom played a special role in the history of physics by providing the key that unlocked the new mechanics that replaced newtonian mechanics.. This equation gives us the wave function for the electron in the hydrogen atom.