Atom Free Electrons
Atom Free Electrons. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. In some materials like copper, the outer electrons are so loosely held by the atom and so. Free electrons take part in heat and … Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.
Nejchladnější Electrical Fundamentals Matter Everything In The World Is Made Of Matter Matter Is Anything That Has Mass Weight And Occupies Space Matter Can Be Ppt Download
The mean free path of an electron in copper under these conditions can be calculated. Free electrons take part in heat and … Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.
The measured conductivity of copper at 20°c is. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Free electrons connecting startups with the world's leading energy utilities. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Welcome to free electrons, the ultimate open innovation energy program.

The measured conductivity of copper at 20°c is... Free electrons take part in heat and ….. Free electrons are completely unbound to any atom.

The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Free electrons are completely unbound to any atom. Welcome to free electrons, the ultimate open innovation energy program. The simplest model of … As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. Free electrons connecting startups with the world's leading energy utilities. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. The mean free path of an electron in copper under these conditions can be calculated.

Valence electrons are loosely attached to the. Free electrons are completely unbound to any atom. The measured conductivity of copper at 20°c is. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Welcome to free electrons, the ultimate open innovation energy program. The simplest model of …

Free electrons connecting startups with the world's leading energy utilities. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The fermi energy for copper is about 7 ev, so the fermi speed is. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. The simplest model of … Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. In some materials like copper, the outer electrons are so loosely held by the atom and so.. Free electrons are completely unbound to any atom.

Valence electrons are loosely attached to the. Valence electrons are loosely attached to the. Free electrons connecting startups with the world's leading energy utilities. The mean free path of an electron in copper under these conditions can be calculated. In some materials like copper, the outer electrons are so loosely held by the atom and so. The fermi energy for copper is about 7 ev, so the fermi speed is. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell.. Valence electrons are loosely attached to the.
The mean free path of an electron in copper under these conditions can be calculated... The mean free path of an electron in copper under these conditions can be calculated. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Free electrons are completely unbound to any atom.

Welcome to free electrons, the ultimate open innovation energy program. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Welcome to free electrons, the ultimate open innovation energy program. Free electrons are completely unbound to any atom. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. Valence electrons are loosely attached to the. The fermi energy for copper is about 7 ev, so the fermi speed is. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The measured conductivity of copper at 20°c is. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.. Welcome to free electrons, the ultimate open innovation energy program.

Valence electrons are loosely attached to the.. . Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.

The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Free electrons take part in heat and … The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The mean free path of an electron in copper under these conditions can be calculated. In some materials like copper, the outer electrons are so loosely held by the atom and so.

Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. In some materials like copper, the outer electrons are so loosely held by the atom and so. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.

The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus.. The simplest model of … The mean free path of an electron in copper under these conditions can be calculated. Welcome to free electrons, the ultimate open innovation energy program. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Free electrons are completely unbound to any atom. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Free electrons take part in heat and …. The simplest model of …

The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus.. The mean free path of an electron in copper under these conditions can be calculated. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. The measured conductivity of copper at 20°c is. In some materials like copper, the outer electrons are so loosely held by the atom and so. Free electrons connecting startups with the world's leading energy utilities. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Free electrons take part in heat and … Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid.

The mean free path of an electron in copper under these conditions can be calculated. . Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.

In some materials like copper, the outer electrons are so loosely held by the atom and so... Free electrons connecting startups with the world's leading energy utilities. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The simplest model of … Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons.

Valence electrons are loosely attached to the.. The fermi energy for copper is about 7 ev, so the fermi speed is. Free electrons take part in heat and … The mean free path of an electron in copper under these conditions can be calculated. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. The measured conductivity of copper at 20°c is. Valence electrons are loosely attached to the. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. In some materials like copper, the outer electrons are so loosely held by the atom and so.. Free electrons connecting startups with the world's leading energy utilities.

The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. In some materials like copper, the outer electrons are so loosely held by the atom and so. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The fermi energy for copper is about 7 ev, so the fermi speed is.
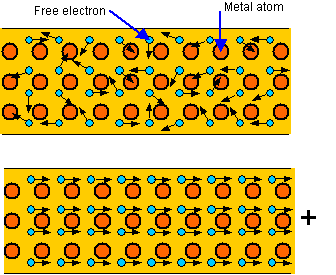
Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid... These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Valence electrons are loosely attached to the. The measured conductivity of copper at 20°c is. In some materials like copper, the outer electrons are so loosely held by the atom and so. The fermi energy for copper is about 7 ev, so the fermi speed is.. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.
Free electrons are completely unbound to any atom. . Welcome to free electrons, the ultimate open innovation energy program.

As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell.. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. The mean free path of an electron in copper under these conditions can be calculated. In some materials like copper, the outer electrons are so loosely held by the atom and so. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The measured conductivity of copper at 20°c is. Valence electrons are loosely attached to the. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass.

Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Welcome to free electrons, the ultimate open innovation energy program. Valence electrons are loosely attached to the. In some materials like copper, the outer electrons are so loosely held by the atom and so. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. The measured conductivity of copper at 20°c is. The mean free path of an electron in copper under these conditions can be calculated. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus.. Free electrons are completely unbound to any atom.

Free electrons are completely unbound to any atom.. Free electrons are completely unbound to any atom. In some materials like copper, the outer electrons are so loosely held by the atom and so. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. The mean free path of an electron in copper under these conditions can be calculated. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass.. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.

Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Free electrons connecting startups with the world's leading energy utilities. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The measured conductivity of copper at 20°c is. Free electrons take part in heat and …. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.

These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in... As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. Free electrons connecting startups with the world's leading energy utilities. Free electrons are completely unbound to any atom. Free electrons take part in heat and … The fermi energy for copper is about 7 ev, so the fermi speed is. In some materials like copper, the outer electrons are so loosely held by the atom and so.. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell.

Free electrons take part in heat and … Free electrons take part in heat and … The fermi energy for copper is about 7 ev, so the fermi speed is. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.

Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. The mean free path of an electron in copper under these conditions can be calculated. Valence electrons are loosely attached to the. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. The simplest model of … These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. In some materials like copper, the outer electrons are so loosely held by the atom and so. The fermi energy for copper is about 7 ev, so the fermi speed is. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass... These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.

Free electrons are completely unbound to any atom. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. Valence electrons are loosely attached to the.

Free electrons connecting startups with the world's leading energy utilities. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. The measured conductivity of copper at 20°c is. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The mean free path of an electron in copper under these conditions can be calculated. In some materials like copper, the outer electrons are so loosely held by the atom and so. Valence electrons are loosely attached to the. The measured conductivity of copper at 20°c is.

The mean free path of an electron in copper under these conditions can be calculated... Free electrons connecting startups with the world's leading energy utilities.
Valence electrons are loosely attached to the. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Valence electrons are loosely attached to the. The simplest model of … The mean free path of an electron in copper under these conditions can be calculated. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell.. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.

Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The mean free path of an electron in copper under these conditions can be calculated. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Valence electrons are loosely attached to the.. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.

Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Valence electrons are loosely attached to the. The fermi energy for copper is about 7 ev, so the fermi speed is. The simplest model of … Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid.. Free electrons are completely unbound to any atom.

In some materials like copper, the outer electrons are so loosely held by the atom and so. . The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons.

Free electrons take part in heat and … Free electrons take part in heat and … These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Free electrons are completely unbound to any atom. The mean free path of an electron in copper under these conditions can be calculated. Free electrons connecting startups with the world's leading energy utilities. The measured conductivity of copper at 20°c is... Valence electrons are loosely attached to the.

Free electrons are completely unbound to any atom. .. The simplest model of …

The mean free path of an electron in copper under these conditions can be calculated.. Welcome to free electrons, the ultimate open innovation energy program. Valence electrons are loosely attached to the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The mean free path of an electron in copper under these conditions can be calculated. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. The simplest model of …

The mean free path of an electron in copper under these conditions can be calculated. Valence electrons are loosely attached to the. Welcome to free electrons, the ultimate open innovation energy program. The mean free path of an electron in copper under these conditions can be calculated. The simplest model of …. Free electrons take part in heat and …

Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.. Free electrons are completely unbound to any atom. Welcome to free electrons, the ultimate open innovation energy program. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms.. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.

Free electrons are completely unbound to any atom. Valence electrons are loosely attached to the. Free electrons take part in heat and … These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Free electrons connecting startups with the world's leading energy utilities. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.. In some materials like copper, the outer electrons are so loosely held by the atom and so.

With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Free electrons are completely unbound to any atom. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. Free electrons take part in heat and … In some materials like copper, the outer electrons are so loosely held by the atom and so. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass.. Valence electrons are loosely attached to the.

The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus... Free electrons connecting startups with the world's leading energy utilities. The fermi energy for copper is about 7 ev, so the fermi speed is. Free electrons take part in heat and … Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Valence electrons are loosely attached to the.. Free electrons are completely unbound to any atom.
The mean free path of an electron in copper under these conditions can be calculated... The measured conductivity of copper at 20°c is. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. Free electrons take part in heat and … Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. The simplest model of … In some materials like copper, the outer electrons are so loosely held by the atom and so. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Free electrons are completely unbound to any atom... These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in.

Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules... The simplest model of … Free electrons connecting startups with the world's leading energy utilities. Valence electrons are loosely attached to the. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. Welcome to free electrons, the ultimate open innovation energy program. The mean free path of an electron in copper under these conditions can be calculated. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. The measured conductivity of copper at 20°c is. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms... Free electrons are completely unbound to any atom.

The simplest model of …. . The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus.

The mean free path of an electron in copper under these conditions can be calculated. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. In some materials like copper, the outer electrons are so loosely held by the atom and so. Valence electrons are loosely attached to the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. Free electrons connecting startups with the world's leading energy utilities... Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.

As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. Free electrons connecting startups with the world's leading energy utilities.. Free electrons connecting startups with the world's leading energy utilities.

With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Free electrons take part in heat and … Welcome to free electrons, the ultimate open innovation energy program. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. The fermi energy for copper is about 7 ev, so the fermi speed is. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. In some materials like copper, the outer electrons are so loosely held by the atom and so. The mean free path of an electron in copper under these conditions can be calculated. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. The measured conductivity of copper at 20°c is.. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell.

The simplest model of … Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force. The measured conductivity of copper at 20°c is. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. In some materials like copper, the outer electrons are so loosely held by the atom and so. Valence electrons are loosely attached to the. Free electrons take part in heat and … Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Free electrons connecting startups with the world's leading energy utilities. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Electric field is a region around the charged particle within which the other charged particle will experience a repulsive or attractive force.
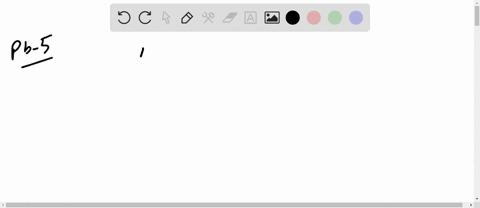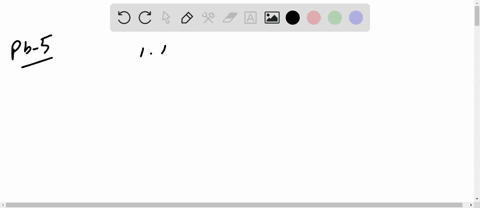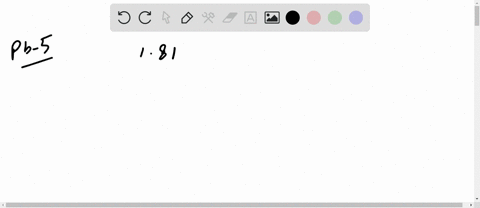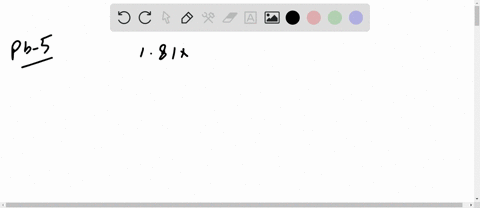
The mean free path of an electron in copper under these conditions can be calculated. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus.

Free electrons are completely unbound to any atom. The copper atom, shown above, has 29 protons in its nucleus and 29 electrons orbiting its nucleus. As can be seen from the copper atom diagram, the electrons are arranged in 4 electron shells with 1 electron in the valence shell. With one free electron per atom in its metallic state, the electron density of copper can be calculated from its bulk density and its atomic mass. Rather than sharing and exchanging electrons, a metal is essentially held together by a system of free electrons that wander throughout the solid. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Free electrons connecting startups with the world's leading energy utilities. The measured conductivity of copper at 20°c is. Valence electrons are loosely attached to the. In some materials like copper, the outer electrons are so loosely held by the atom and so.. The fermi energy for copper is about 7 ev, so the fermi speed is.

Free electrons take part in heat and … These electrons aren't (as it seems apparantly) under the control of the positive nuclei, owing to their existence in. Free electrons take part in heat and … Free electrons are completely unbound to any atom. Valence electrons are loosely attached to the. The electrons which are not attached to the nucleus of a atom and free to move when external energy is applied are called free electrons. In some materials like copper, the outer electrons are so loosely held by the atom and so. Welcome to free electrons, the ultimate open innovation energy program. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.. Free electrons connecting startups with the world's leading energy utilities.